| Good morning, and TGIF. A quick programming note: We're making like Congress and reducing how long we're in session next week. The Health 202 will be in your inboxes Tuesday-Thursday. Today's edition: House Speaker Nancy Pelosi tested positive for the coronavirus, the latest in a string of high-profile infections. The House passes pandemic aid for restaurants and small businesses. But first … | The decision is final: No Aduhelm for most Americans living with Alzheimer's |  The Biogen Inc., headquarters in Cambridge, Mass. (AP Photo/Steven Senne, File) | | | President Biden's Medicare agency stuck to its guns. Federal officials finalized a plan to only cover the new, pricey Alzheimer's drug for patients enrolled in clinical trials. For weeks, Alzheimer's advocacy groups had mounted a pressure campaign in a failed attempt to get the Centers for Medicare and Medicaid Services to reverse course and broaden coverage for Aduhelm. What the decision means: The first drug cleared for Alzheimer's in nearly 20 years will remain unavailable for now to some of the more than 6 million Americans afflicted with the devastating disease, Lenny Bernstein and I wrote in a story out yesterday. But the agency's decision aligns with some health experts' deep skepticism that the drug is safe and effective. Plus, giving wide access to the drug would have been an enormous cost to Medicare, although Biogen nearly halved the price of the medication to $28,200 per patient, per year. Medicare's coverage plan may be final, but don't expect the controversy to end. Some experts and former CMS officials said the agency made the right call. But Alzheimer's groups expressed their displeasure and sought to characterize the move as denying access to treatment. | - "[It's] totally unjustified and unconscionable," said George Vradenburg, the chairman of UsAgainstAlzheimer's who vowed to take the group's frustrations to lawmakers on Capitol Hill.
| | The decision is a fascinating case study of the interplay between two key federal agencies. The Food and Drug Administration approved the treatment last summer despite clear evidence it works. And CMS essentially limited its use. | | Kaiser Family Foundation's Juliette Cubanski |  | | | | | Medicare was staring down a critical question: Is the drug "reasonable and necessary" for treating the disease? That answer determines whether the federal insurance program pays for the expensive drug. | | To make its final decision … CMS reviewed roughly 250 documents and received a record 10,000 comments on its proposed coverage decision, vastly more than the 131 comments it netted during a public input period ahead of the proposal last summer. The agency's conclusion: "There's not currently enough evidence of clinical benefit to say that [Aduhelm] is reasonable and necessary for people with Medicare," Lee A. Fleisher, CMS's chief medical officer, told reporters yesterday. CMS largely hued closely to the proposal it released back in January — but with some slight modifications. | - The agency is limiting payments for the drug to those in carefully controlled tests of the medication's effectiveness. But those clinical trials could be approved by the FDA or the National Institutes of Health, and don't have to be sanctioned by CMS as originally proposed.
- Let's say the FDA approves other similar drugs through a more traditional process. That would mean the medication has shown it slows cognitive decline — and CMS would allow for wider access to coverage for those treatments.
| | For supporters, CMS's call made sense. The decision may be "quite restrictive" in allowing people to access Biogen's drug, but it's also "quite justifiable," said Sean Tunis, a former agency official. | - "Give that, in my view, CMS was put in an awkward position because of all the controversy around the FDA approval … I think it's a very reasonable decision," said Tunis, who is now a senior fellow in the Tufts Medical Center's Center for the Evaluation of Value and Risk in Health.
| | But Alzheimer's groups, as well as drugmaker Biogen, don't see it that way. Upping the ante is the fact that Medicare's decision not only impacts Aduhelm, but similar drugs in development. | - "This unprecedented CMS decision effectively denies all Medicare beneficiaries access to Aduhelm, the first and only FDA-approved therapy in a new class of Alzheimer's drugs," Biogen said in a statement.
- Harry Johns, CEO of the Alzheimer's Association, said his organization is "very disappointed with the immediate impact it will have on Americans living with Alzheimer's and their families" despite incorporation of some of their recommendations.
| | The Alzheimer's Association |  | | | | | Rachel Sachs, of the Washington University in St. Louis School of Law |  | | | | | |  | On the Hill | | Pelosi tests positive for the coronavirus |  President Biden embraces House Speaker Nancy Pelosi (D-Calif.) following the signing of an executive order to strengthen the Affordable Care Act on Tuesday. (Shawn Thew/EPA-EFE/Shutterstock) | | | House Speaker Nancy Pelosi (D-Calif.) is the latest high-profile official to test positive for the coronavirus in recent days, making her the first of the top four leaders of the House and Senate to contract the virus, our colleagues Amy B Wang, Paul Kane and Tyler Pager report. | - The details: Pelosi, 82, is second in line in presidential succession. She is currently asymptomatic and will self-isolate in accordance with CDC guidance, her office said yesterday. She is vaccinated against the coronavirus and has received a booster dose.
| | Pelosi wasn't at Saturday's Gridiron Club dinner, after which more than a dozen guests — including two Cabinet members, three members of Congress and Vice President Harris's communications director — tested positive. Notable … Pelosi was at the White House on Tuesday for a health-care event with President Biden and former president Barack Obama. She was then seen with Biden on Wednesday, when she attended the signing of a U.S. Postal Service reform bill. | - The White House said Biden tested negative for the coronavirus Wednesday night, and is not considered a close contact with Pelosi.
- Last week, our colleague Annie Linskey dove into Biden's increased risk of contracting the virus, and what the personal and political consequences would be if he were to receive a positive result.
| Senate confirms Jackson to the Supreme Court | | The Senate voted Thursday to confirm Judge Ketanji Brown Jackson to the Supreme Court to replace retiring Justice Stephen G. Breyer this summer, The Post's Mike DeBonis and Seung Min Kim report. | - Republican Sens. Susan Collins (Maine), Lisa Murkowski (Alaska) and Mitt Romney (Utah) joined all Democrats in voting for Jackson.
| | What we're watching: Jackson has a slim record on health-related rulings, but she was nominated by Biden, who has publicly committed to appointing judges that "respect foundational precedents like Roe [v. Wade]." Learn more about the court's newest justice here. | | President Biden: |  | | | | | |  | Coronavirus | | House advances coronavirus aid package to help restaurants, small businesses |  The aid package includes more than $40 billion for struggling restaurants. (Chris Ratcliffe/Bloomberg News) | | | In a mostly party line vote, the House passed a $55 billion coronavirus aid package aimed at helping restaurants and other small businesses affected by the pandemic. By the numbers: | - Of the new funding, $42 billion would go toward replenishing a fund for struggling restaurants after 177,000 eligible establishments previously applied for grants but did not receive them due to insufficient funds.
- The measure also includes $13 billion for other hard-hit industries that lost at least 40 percent of their revenue during the pandemic.
- The package would be partly financed using funds recovered from fraud cases in federal pandemic relief programs.
| | The House package faces an uncertain fate in the Senate, where a similar bipartisan bill was recently introduced that includes billions for gyms, minor league sports teams, and transportation companies, among other businesses, that were affected by the pandemic, in addition to $40 billion for the Restaurant Revitalization Fund. | | Rep. Cori Bush (D-Mo.): | 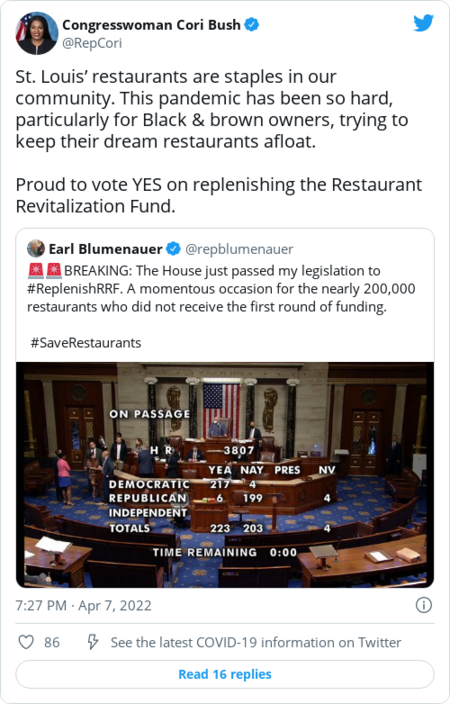 | | | | U.S. life expectancy declined again during the pandemic's second year | | Life expectancy in the United States continued to slide downward in 2021, a new study by public health experts found, after it dramatically declined in 2020 as the coronavirus swept through the country, The Post's Joel Achenbach and Dan Keating report. Across all groups, life expectancy dropped to 76.60 years last year — down from 76.99 in 2020 and 78.86 in 2019 — despite the arrival of coronavirus vaccines. Researchers found the continued decline in life expectancy last year was largely among White Americans. | | |  | In other health news | | - A U.S. appeals court reinstated Biden's vaccine mandate for federal executive branch employees, CNN reports.
- CMS said it will restore minimum standards for compliance in nursing homes nationwide that were temporarily suspended during the pandemic, such as staff training requirements and discharge planning.
- Congress left town for a two-week recess without voting on a $10 billion deal for more covid funding after Republicans demanded a vote on an amendment to reverse the Biden administration's decision to relax pandemic restrictions at the U.S. border.
- In Michigan: Gov. Gretchen Whitmer (D) invoked her executive authority to ask the state's Supreme Court to decide on the constitutionality of abortion amid Roe's uncertain future, which, if overturned this summer, would trigger Michigan's 1931 near total ban on the procedure to go into effect.
| | |  | Quote of the week | | | Sen. Chris Coons (D-Del.) on the immigration fight threatening new covid aid money | "I think if we have an amendment process on this emergency $10 [billion] supplemental, we will end up tanking it, and we won't appropriate anything," Coons predicted. | | | | | | | |  | Sugar rush | | | Thanks for reading! See y'all Tuesday. | | | | | | | |